200, 300 workshop technical transformation and construction project environmental impact assessment report draft publicity
Release time:
2023-12-11
According to the the People's Republic of China Environmental Impact Assessment Act (2018Year12Month29Day), Environmental ImpactMeasures for the Evaluation of Public Participation (2019Year1Month1Day) of the relevant provisions, now carried out200,300Workshop technical transformation and construction project environmental impact assessment report information publicity, to solicit the opinions and suggestions of the general public.
1.Name and summary of construction project
1Project Name:200,300Workshop Technical Transformation Construction Project
2Project Location:Shandong Anxin Pharmaceutical Co., Ltd. Existing Factory(Wenliang Road, Dongjia Street, Licheng District10678No.)
3Main construction contents:This project is for existing10In the production workshop.4A workshop (200Workshop and300Workshop2API production workshop,2000Workshop and2300Workshop2preparation production workshop) to implement the reconstruction;500The workshop retains the production facilities, and the workshop stops production and is idle. Existing100API production workshop and2100the workshop,2500the workshop,2600Workshop and2800Workshop, etc.4There is no change in the production workshop.
After the project was put into production, some production workshops formed a situation of co-production of multiple products, which improved the types of products produced, and enhanced the market competitiveness and risk resistance of enterprises,Can produce azithromycin, alprazolam, montelukast sodium and other API products10species and lyophilized raw powder (tazobactam sodium piperacillin sodium).
4Nature of Project:Alteration
5Overview of existing works:Shandong Anxin Pharmaceutical Co., LtdExisting works are mainly100the workshop,200the workshop,300the workshop,500the workshop,2000the workshop,2100the workshop,2300the workshop,2500the workshop,2600the workshop,2800Workshop, total production of APIs7species, freeze-dried preparations and freeze-dried raw powders.(tazobactam sodium piperacillin sodium). Existing projects are implemented environmental protection "three simultaneous" system, has obtained a permit, the certificate number is:91370100MA3QGRX746001P.
2.Name and contact information of construction unit
Construction unit: Shandong Anxin Pharmaceutical Co., Ltd.
Contact: Mr. Chen
Contact Phone:0531-83128097
Electronic E-mail:zhu.chen@qilu-pharma.com
Zip Code:250100
3.Name of the entity preparing the environmental impact report
Evaluation Unit: Shandong Unate Environmental Technology Co., Ltd.
4.The network link of the full text of the draft of the environmental impact report and the ways and means of consulting the paper report.
(1)Links to the draft environmental impact report of the Project
Link: https://pan.baidu.com/s/1NRStK3WyI3lqeGxzxgRi4g?pwd=pwth
(2) If you need to check the paper version of the project environmental impact report, please contact the construction unit during the publicity period, and the public is welcome to call and inquire.
5.Public Scope of Consultation
Residents around the project and outside the project boundary5kmScopewithinsocial groups,Residentsand individualsWait.
6.Web Link to Public Opinion Form
https://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/201810/t20181024_665329.html
7.Ways and means of public input
The public can submit the completed public opinion questionnaire to the construction unit within the specified time by sending letters, e-mails, etc. to the designated contact person of this publicity.
8.Starting and ending time for public comments
The publicity time is2023Year12Month11Day2023Year12Month15Day, from the date of publication of the information5Valid within working days.
Shandong Anxin Pharmaceutical Co., Ltd
2023Year12Month11Day
Related Annexes
Latest developments

Shandong anxin pharmaceutical co., ltd. entrusted Shandong unate environmental technology co., ltd. to compile the environmental impact report form of the laboratory and quality inspection central control room renovation project in November 2023, and obtained the environmental impact assessment approval through the municipal bureau review on April 19, 2024, with the approval number: jilihuan report form [2024] no 13.

Shandong anxin pharmaceutical co., ltd. entrusted Shandong unate environmental technology co., ltd. to compile the environmental impact report of 200 and 300 workshop technological transformation construction project in December 2021, and obtained the environmental impact assessment approval through the municipal bureau review on February 6, 2024, with the approval number: economic and environmental report [2024] no 4.
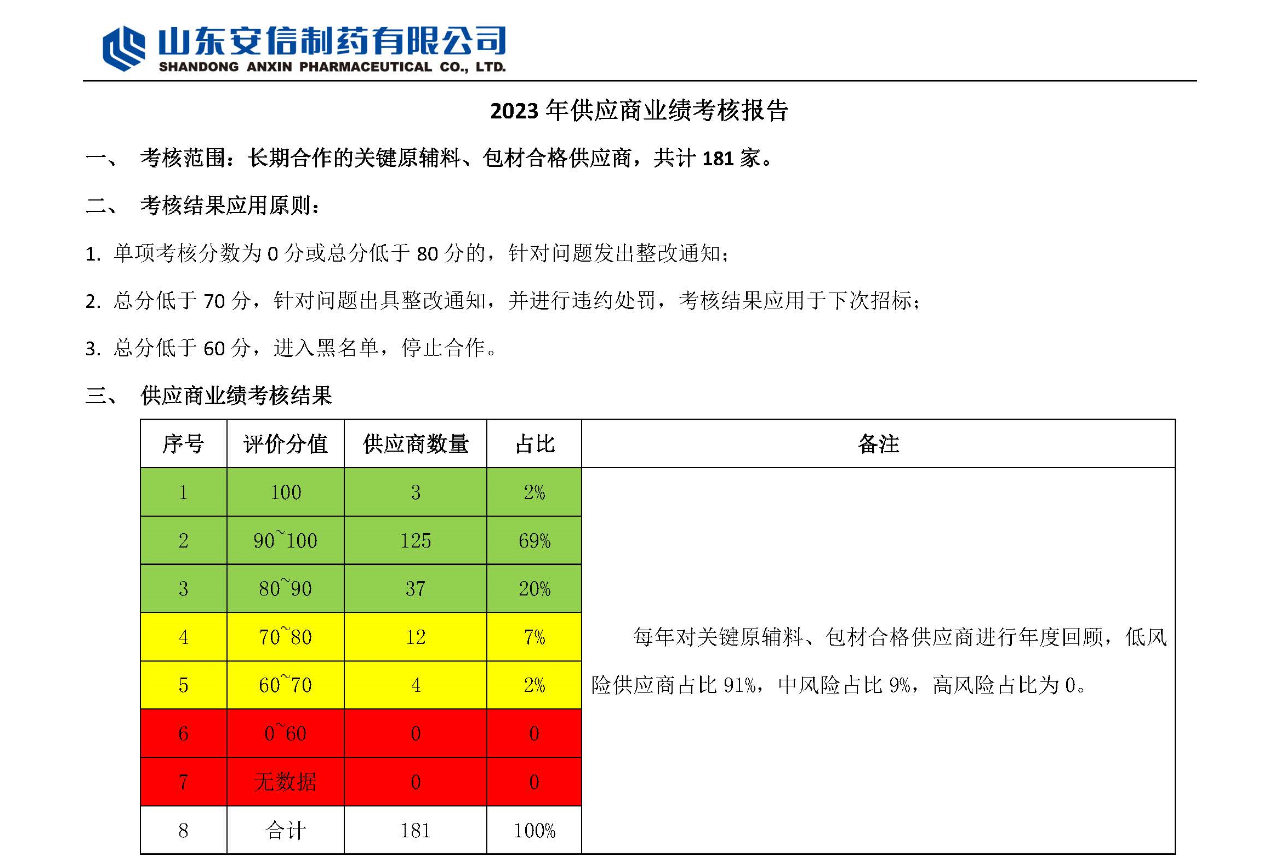
2023 Shandong Anxin Pharmaceutical Co., Ltd. Supplier Performance Evaluation Report
2023 Shandong Anxin Pharmaceutical Co., Ltd. Supplier Performance Evaluation Report
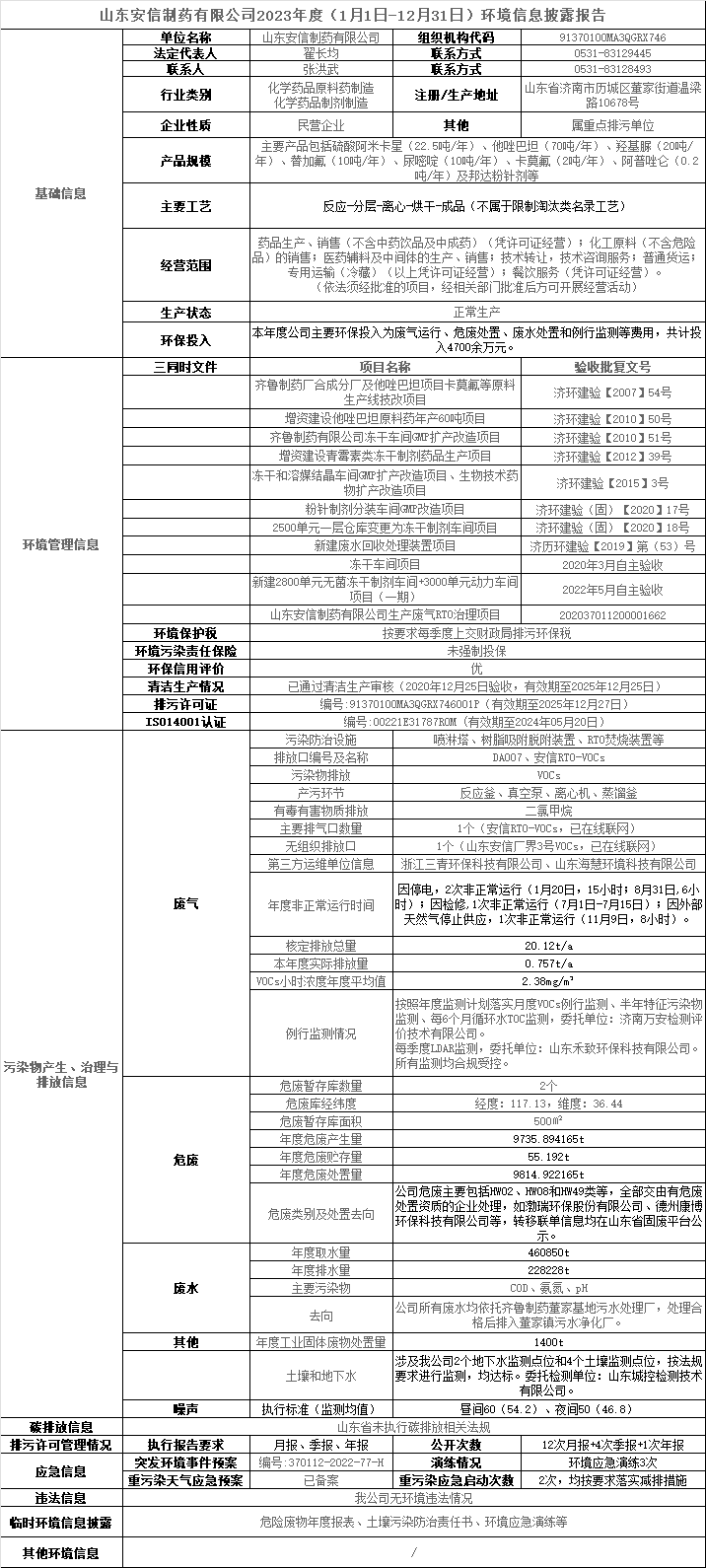
Environmental Information Disclosure Report of Shandong Anxin Pharmaceutical Co., Ltd. for 2023 (January 1-December 31)
Information Disclosure of Energy Saving and Carbon Reduction of Shandong Anxin Pharmaceutical Suppliers